Thermodynamics Study for Selective Leaching of Copper and Zinc from Complex Secondary Raw Materials Using Oxidative Sulfuric Acid Leaching
DOI:
https://doi.org/10.30544/MMD10Abstract
This study focuses on evaluating the optimal process conditions for selectively leaching copper and zinc from complex secondary raw materials using the oxidative sulfuric acid leaching process. The research includes both physical and chemical characterization of the materials and a comprehensive thermodynamic analysis to assess the thermodynamic properties and behaviors of the system under investigation.
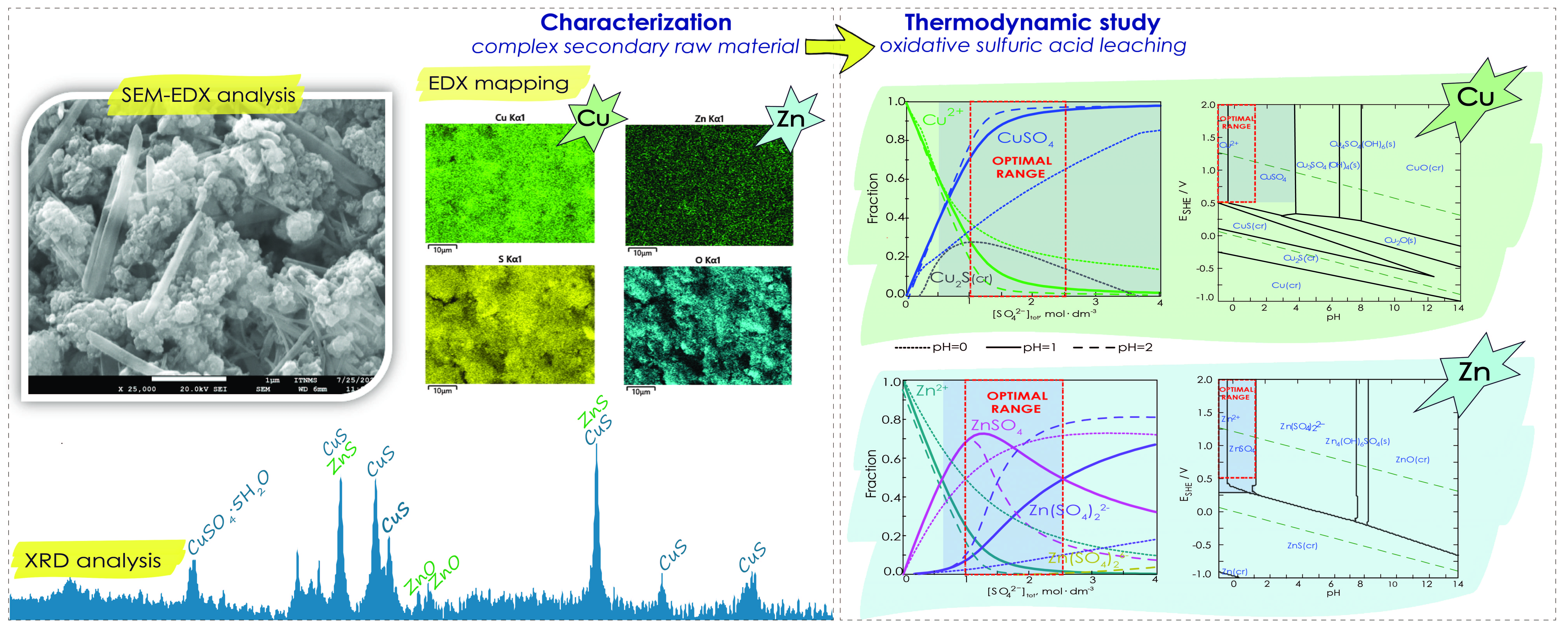
The physical characterization involves determining parameters such as bulk density, moisture content, and granulometric composition of the samples. Elemental composition analysis is performed using X-ray fluorescence spectrometry (XRF) and energy dispersion spectroscopy (EDX), while the phase composition is determined through X-ray diffraction (XRD) analysis. Additionally, the materials' morphology is examined using scanning electron microscopy (SEM).
Fraction diagrams are created using Hydra/Medusa software to assess the phase distributions of zinc and copper during the leaching process. The stability of the metals and potential chemical reactions within a defined temperature range is analyzed using Eh-pH diagrams with the HSC 9 Chemistry Software.
Based on the thermodynamic analysis conducted, it is determined that effective leaching of copper and zinc from the complex secondary raw materials is achievable. Furthermore, the results indicate that the leaching process is relatively insensitive to temperature variations. The optimization of the leaching process should primarily focus on achieving optimized consumption of the oxidizing agent.
Keywords:
Thermodynamics, Leaching, Copper, ZincReferences
Ahmed, I.M., A.A. Nayl, and J.A. Daoud. "Leaching and Recovery of Zinc and Copper from Brass Slag by Sulfuric Acid." Journal of Saudi Chemical Society 20 (2016) s280-s285.
Antonijević, M.M., M.D. Dimitrijević, Z.O. Stevanović, S.M. Serbula, and G.D. Bogdanovic. "Investigation of the Possibility of Copper Recovery from the Flotation Tailings by Acid Leaching." Journal of Hazardous Materials 158, no. 1 (2008): 23-34.
Asadi, Tahereh, Asghar Azizi, Jae-chun Lee, and Mohammad Jahani. "Leaching of Zinc from a Lead-Zinc Flotation Tailing Sample Using Ferric Sulphate and Sulfuric Acid Media." Journal of Environmental Chemical Engineering 5, no. 5 (2017): 4769-75.
Cao, Lei, Ya-long Liao, Gong-chu Shi, Yu Zhang, and Mu-yuan Guo. "Leaching Behavior of Zinc and Copper from Zinc Refinery Residue and Filtration Performance of Pulp under the Hydrothermal Process." International Journal of Minerals, Metallurgy, and Materials 26, no. 1 (2019): 21-32.
Chen, Tao, Chang Lei, Bo Yan, and Xianming Xiao. "Metal Recovery from the Copper Sulfide Tailing with Leaching and Fractional Precipitation Technology." Hydrometallurgy 147-148 (2014): 178-82.
Kamberović, Željko, Milisav Ranitović, Marija Korać, Zoran Andjić, Nataša Gajić, Jovana Djokić, and Sanja Jevtić. "Hydrometallurgical Process for Selective Metals Recovery from Waste-Printed Circuit Boards." Metals 8, no. 6 (2018): 441.
Karimov, K. A., S. S. Naboichenko, A. V. Kritskii, M. A. Tret'yak, and A. A. Kovyazin. "Oxidation Sulfuric Acid Autoclave Leaching of Copper Smelting Production Fine Dust." Metallurgist 62, no. 11-12 (2019): 1244-49.
Nozhati, Ramezan Ali, and Asghar Azizi. "Leaching of Copper and Zinc from the Tailings Sample Obtained from a Porcelain Stone Mine: Feasibility, Modeling, and Optimization." Environmental Science and Pollution Research 27, no. 6 (2019): 6239-52.
Puigdomenech, I. HYDRA (Hydrochemical Equilibrium-Constant Database) and MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) Programs. Royal Institute of Technology, Stockholm, 200, Link
Roine, A., and P. Lamberg. "HSC Chemistry, vers. 9." Outotec Pori (2020). Link
Santos, Sílvia M.C., Remígio M. Machado, M. Joana Correia, M. Teresa Reis, M. Rosinda Ismael, and Jorge M.R. Carvalho. "Ferric Sulphate/Chloride Leaching of Zinc and Minor Elements from a Sphalerite Concentrate." Minerals Engineering 23, no. 8 (2010): 606-15.
Wang Ben, Meng Yun. Recovery method of copper and zinc oxide from waste copper-zinc catalyst, Patent: CN1258752A (1998). Link
Zhang Xiaoyang, Hu Zhibiao, Ling Hua Zhao, Hu Gaorong, Qiu Chuangui, Liu Jinglin, Li Qian, Huang Hong, Xu Xiaofeng. Application method of waste copper-based catalyst to preparing catalyst for preparing hydrogen from methanol, Patent: CN102125851B (2010). Link
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Nataša Gajić, Milisav Ranitović, Miloš Marković, Željko Kamberović

This work is licensed under a Creative Commons Attribution 4.0 International License.